In global manufacturing, technical documentation is no longer a “product accessory.” It is a risk asset that directly affects regulatory compliance, legal exposure, and time-to-market. For organizations managing 50+ languages at scale, even small documentation errors can trigger customs delays, forced rework, product holds, recalls, or expensive legal consequences.
At Hansem Global, we combine 35 years of technical documentation expertise with an ISO 9001-based Quality Management System (QMS) and rigorous data analysis. We do not treat manuals as “deliverables.” We treat them as an operational control system—built to reduce risk and optimize total cost of ownership (TCO) across the full documentation lifecycle.
Accuracy Alone Is Not Enough
Documentation quality is often evaluated through usability—clarity, consistency, and user experience. Those factors matter—but in regulated manufacturing, the most costly failures are often compliance- and release-blocking defects that sit outside traditional usability checks. Common examples include:
- Missing mandatory regulatory statements
- Incorrect country-specific disclosures
- Incorrect version, revision, or issue date labeling
- Specification and release-metadata errors that can fail audits or delay shipment
That is why Hansem Global treats regulatory-related documentation defects as a priority category. We capture every defect found during internal audits, classify it by type, and track performance monthly as well as year to date.
We apply Pareto analysis to select the Top 3 most frequent error categories and eliminate them systematically.
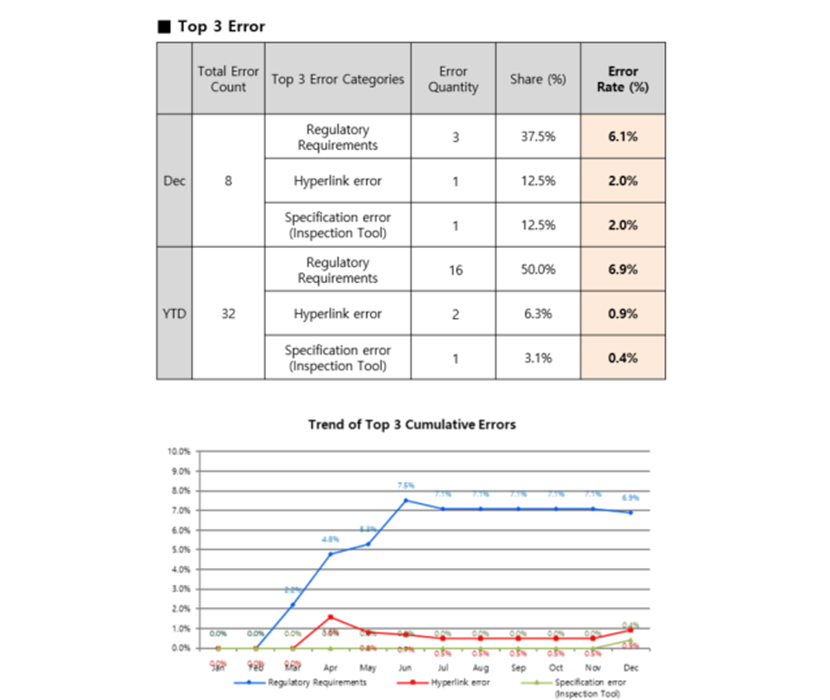
In one monthly quality report example (December), “Regulatory Requirements” accounted for 37.5% of source (English) manual errors. On a year-to-date basis, that category approached 50%.
The implication is clear: regulatory risk is not a variable that suddenly appears in translation. It is structural. If it is not controlled at the source-content stage, it cannot be reliably controlled downstream—especially across dozens of languages, formats, and release cycles.
Managing Quality with Evidence, Not Assumptions
Documentation quality does not hold at scale because a team “feels confident” it is fine. In high-cost environments—global launches, synchronized multi-market releases, and regulated product categories—quality must be managed as a provable state.
That is why an evidence-based audit trail is essential. Hansem Global applies a monthly analysis framework to quantify documentation quality and connect the results to a continuous improvement cycle. We do not only try to “reduce the number of errors.” We manage quality against informing KPIs and trend data, and we execute three core practices:
- Target-Based KPI Control
We set an explicit quality target line (for example, 6.4%) and measure performance against it. We monitor both monthly results and year-to-date trends to stabilize quality below the target threshold. - Trend Management, Not One-Time Numbers
We evaluate both monthly variation and cumulative trend lines. This helps us distinguish a one-time fluctuation from a structurally recurring issue—and respond with the appropriate corrective action. - Continuous Improvement Under Load
Even in high-volume periods (for example, May), we track risk through data and keep year-to-date nonconformance stable (for example, at 2.4%). This is how process robustness is proven—not claimed.
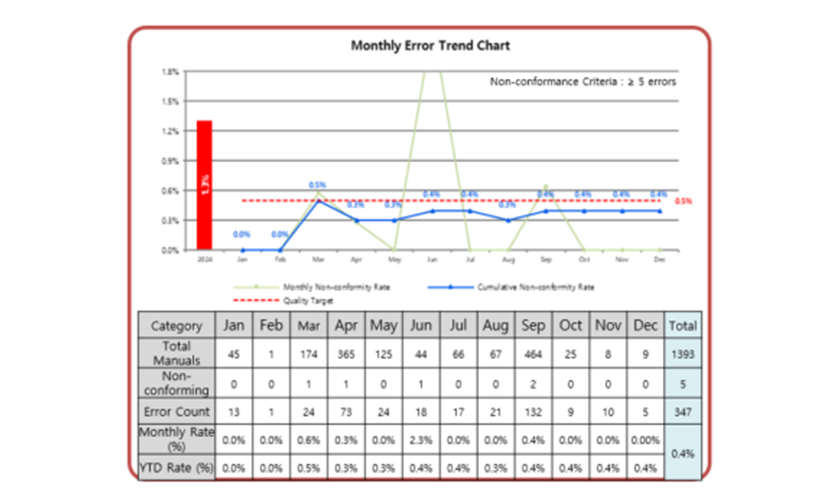
How we define the KPI
- A “nonconformance” is a defect state that meets a defined scoring threshold (e.g., 6 points or higher) based on items directly tied to release risk.
- The “nonconformance rate” is calculated as: number of manuals judged nonconforming (numerator) / total manuals inspected that month (denominator).
- The “target line” (e.g., 6.4%) is an operational KPI used to ensure that the cumulative trend remains reliably below the threshold—even when monthly results fluctuate.
Data-Driven Quality Metrics: We visualize monthly and cumulative nonconformance trends against the target line to prove stability of the quality control system in real time.
Do Not Stop at “Fixing” Errors
Stabilizing quality is not about fixing errors quickly. It is about preventing the same class of error from returning. The goal is recurrence prevention through process improvement.
To do that, Hansem Global operates two quality tracks:
- Track 1: Errors found in internal inspection and QA
- Track 2: Escaped defects (“leakage”) found during customer review or engineering/developer review
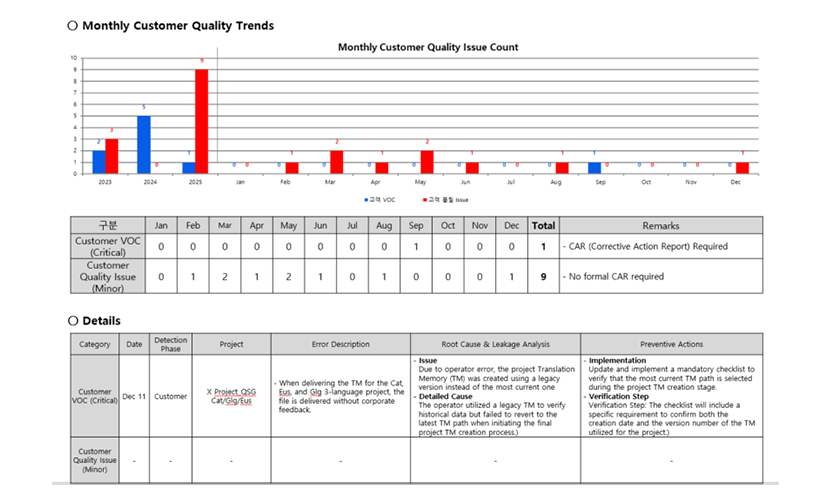
Leakage is treated as a critical management signal. We classify leaked issues by severity and apply structured root cause analysis to build prevention—not just correction. We treat escaped defects found at customer/developer review as a key metric at the final stage of the production workflow, and we monitor them closely.
Severity-Based Response Discipline
We separate leakage into:
- Customer VOC (Critical): cases where the customer requires formal corrective action reporting
- Customer Quality Issue (Minor): cases where the customer does not require a formal countermeasure report
This classification determines response priority and the control level applied.
CAPA and Root Cause Closure
When a defect is found, we trace why it passed internal filters—where it escaped, and why. Then we implement system-level countermeasures through CAPA (Corrective and Preventive Action). A single human error must lead to a stronger system: updated checklists, improved version-control rules, and tighter TM application protocols—so recurrence becomes structurally difficult.
Leakage analysis makes accountability visible and drives a recurrence-prevention improvement loop—where one human error results in checklist and version-control protocol upgrades.
Quality Control Is Not Overhead. It Lowers TCO.
An evidence-based documentation quality system creates business value beyond the abstract goal of “better quality.” It reduces operating cost and release risk in measurable ways:
- Operational visibility: Monthly reports quantify current quality, top risk categories (Top 3), and improvement impact.
- Independent verification: A dedicated QA function audits outputs with independence from production teams.
- Traceable prevention: Escaped defects are investigated to the end, and causes are closed with prevention controls.
- Global consistency at scale: Even across 50+ languages, regulatory compliance and terminology consistency are managed under the same source-standard rules.
- Lower documentation TCO: Post-release corrections, re-issuance costs, and regulatory risk costs are prevented earlier—reducing total lifecycle cost.
A Documentation Quality Partner for Global Releases
For manufacturers operating multilingual documentation at scale, what you need is not a translation supplier. You need a documentation quality partner that can manage regulatory risk and protect brand integrity through a provable quality control system.
Hansem Global brings 35+ years of documentation expertise, an ISO 9001-based QMS, and evidence-based quality analytics to help global manufacturers release documentation with confidence—any product, any market, any language—under a system that keeps quality in a demonstrably controlled state.