Boehringer Ingelheim is a German pharmaceutical company based in Ingelheim, Germany. It was founded in 1885 by Albert Boehringer when he purchased a small chemical plant in Ingelheim am Rhein. As one of the top 20 pharmaceutical companies worldwide, it has 145 branches across the globe with over 51,000 employees. Its products include prescription drugs, over-the-counter drugs, animal drugs, and biopharmaceutical drugs. It is also strengthening its position as the number two company in the world in the animal drug industry by preventing and treating the diseases of pets and livestock.
Hansem Global has been offering multi-language translation services for promotional videos, package inserts, clinical trial documents, lay summaries for the public, etc. for Boehringer Ingelheim.
The Industrial Growth of Pharmaceutical Companies with the Advent of the Aging Society
The aging of society and the development of the modern medical industry will inevitably provoke high growth in the pharmaceutical industry. Along with the lengthening of the human lifespan, the number of people with chronic diseases is rapidly increasing, requiring pharmaceutical companies to put a lot of effort and investment into the development of new medicine to combat diseases that currently lack any suitable medicine, allowing people to lead healthy lives.
Hansem Global has continuously offered translation services that enabled the provision of medicine to approximately 3,000 patients in the year 2022, including “JARDIANCE®,” an antidiabetic medication for people with diabetes, which is a typical chronic disease among senior citizens, and “OFEV,” a medication for certain pulmonary conditions which have proliferated since the COVID-19 pandemic. In particular, JARDIANCE is appraised to have maximized its commercial value by expanding indicators for heart failure treatment in addition to type 2 diabetes.(Source: https://www.medifonews.com/news/article.html?no=178872)
Establishment of Local Hansem Global Subsidiary
The following diagram shows the market share of pharmaceutical sector worldwide as reflected in ten major countries in 2022, in which the United States has positioned itself on top overwhelmingly, followed by China. South Korea also ranked in the top ten.
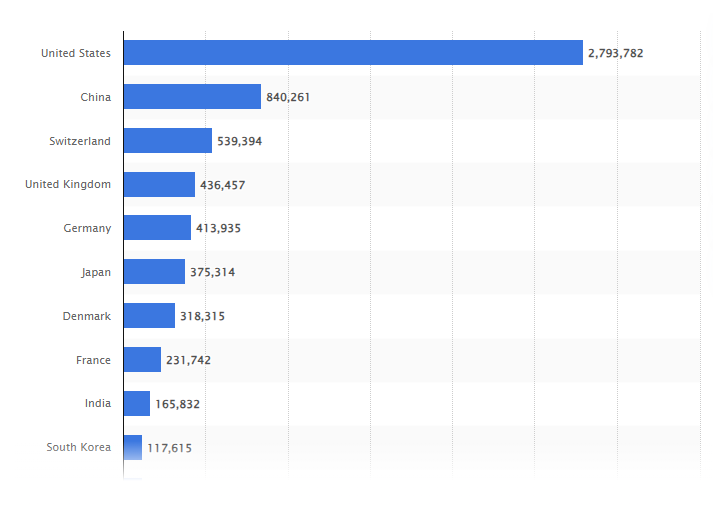
As a result, Hansem Global established a branch in North America in 2018, aimed at serving life sciences and pharmaceutical companies in the US. This US subsidiary has adeptly executed numerous projects for life sciences and pharmaceutical firms, enhancing its linguistic expertise tailored to various therapeutic domains and clinical trials. Furthermore, Hansem Global has secured the ISO27001 Information Data Security Management System certification, ensuring robust protection of client data.
Additionally, Hansem Global’s Korean branch recently secured its first Global Regulatory Affair project from Boehringer Ingelheim, a company it has an ongoing partnership with. This project involved reviewing a Korean-English package insert of about 100,000 words, and was successfully completed with the help of specialized SME reviewers assigned by Hansem. Moreover, Hansem Global has expanded its presence by establishing local subsidiaries in Vietnam and China, where they have been successful in acquiring projects from numerous international pharmaceutical companies.
The Importance of a Proper Localization Strategy in the Pharmaceutical Industry
A systematic localization strategy is required for global pharmaceutical companies to enter various markets successfully because the barriers to expansion are relatively high compared to other industries. As the pharmaceutical industry is directly connected to national health, local government regulations must be complied with throughout the whole process from production and distribution to sales, making comprehensive understanding of the local regulations essential. The local people’s culture and preferences need to be considered as well. If a company falls behind in sales activities or promotions, even the “mega hit” products will also stumble in that country. Good examples of this are Kyung Nam Pharm and Kwang Dong Pharmaceutical, who failed to settle into the Chinese market even with Lemona, Ssanghwa-tang, and Vita 500, which are highly successful in Korea, at the forefront.
As for Boehringer Ingelheim Korea, they are engaged in various activities domestically, such as expanding their product line with nonalcoholic steatophepatitis (NASH) medicine as a joint venture with Yuhan, a domestic company and engaging in 1:1 meetups with promising domestic startups to support their global expansion.(Source: https://weekly.donga.com/science/article/all/11/94918/1)
Market Expansion of Domestic and Overseas Pharmaceutical Companies
As mentioned above, a “localization strategy” for understanding the distinct characteristics and culture of the local market is essential for pharmaceutical companies when entering into a market, whether it’s domestic or overseas. Following local laws and regulations is mandatory. Likewise, it is necessary to correspondingly demonstrate a commitment to ethics through continuous CSR activities to be perceived as a pharmaceutical company that takes responsibility for people’s health. As the pharmaceutical industry is subject to many restrictions, the companies are required to make a lot of preparations in order to pass the high standards of the local food and drug safety administrations of the country they wish to enter into.
Hansem Global is equipped with a multi-language resource pool that is specialized for medical documentation, medical equipment manuals, and clinical trials. Professional SMEs with long-term experience in clinical trial documents with qualifications of the highest standards are specially assigned to each project to provide high-quality translations for global pharmaceutical companies. In addition, it has provided translation services in European languages for over 20 reagents and medical supply manuals of global pharmaceutical manufacturing companies and has experience in translating various types of documents, such as product brochures and material safety data sheets (MSDS).
Hansem Global’s Support in Successful Localization for the Global Pharmaceutical Company, Boehringer Ingelheim
Hansem Global has been steadily ranked as a top 100 localization expert worldwide by CSA Research, a localization research institute in the US, and is the only Korean company that has made the cutoff. Hansem Global is a language service provider who has also established a quality management process in conformity with international standards through acquiring ISO17100 certification for translation services and was the first Asian company to obtain the certificate.
Hansem Global has long proven its value as a specialized localization expert with a history spanning more than 30 years. It organized a resource team that specializes in the medical and dental industries in 2017 that provides full multi-language localization services, providing services ranging from English composition to translation and desktop publishing (DTP) for multiple global pharmaceutical companies. QA configuration has been reinforced autonomously to avoid mistranslations of terminology, precise dosages, or figures as they are directly connected to the life and well-being of patients impacted by the documentation of those medicines.
Hansem Global is always striving to provide accurate and highly readable information to users worldwide by complying with our customers’ requirements and restrictions through inquiries and discussions done in advance so that consumers can use the products safely.






