In the complex landscape of medical devices, ensuring clear and accurate communication is not just a matter of convenience; it’s a critical component of patient safety and regulatory compliance. “Instructions for Use” (IFU) serve as the cornerstone of guidance for healthcare professionals and patients navigating the intricacies of device operation and maintenance. However, translating IFU presents formidable challenges, demanding meticulous attention to detail and precision to avoid potentially dire consequences. In this comprehensive exploration, we delve into the indispensable role of the back translation process in upholding the integrity and accuracy of translated IFU for medical devices.
Understanding the Complexity
The translation of IFU for medical devices transcends mere linguistic proficiency; it encompasses a multifaceted terrain of technical jargon, regulatory standards, and cultural considerations. The ramifications of even the slightest mistranslation or ambiguity within IFU can be profound, ranging from compromised patient safety to regulatory non-compliance, and the stakes are exponentially higher in the realm of healthcare. Thus, a robust translation process is imperative to navigate these complexities and mitigate risks effectively.
Key Aspects of Back Translation
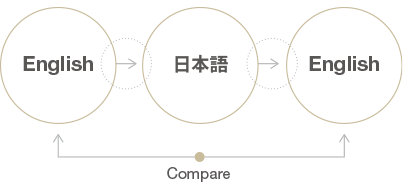
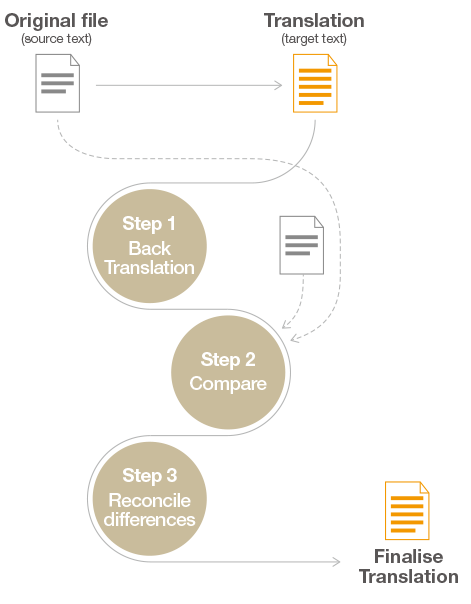
At the heart of ensuring the fidelity of translated IFU lies the back translation process—a rigorous quality assurance measure designed to validate the accuracy and coherence of the translated content. This iterative process involves translating the document back to the source language by an independent translator who was not privy to the original text. By comparing the back-translated version with the original, any discrepancies or errors can be promptly identified and rectified, thereby fortifying the integrity of the translated IFU.
Ensuring Accuracy and Consistency
The deployment of back translation serves as a bulwark against potential inaccuracies and inconsistencies within translated IFU. By subjecting the translated content to meticulous scrutiny through the lens of back translation, manufacturers can preemptively identify and address any deviations from the original text. This meticulous attention to detail is indispensable in upholding the highest standards of accuracy and consistency, particularly in the context of medical device instructions where precision is paramount.
Regulatory Compliance
In the highly regulated landscape of medical devices, adherence to stringent regulatory requirements is non-negotiable. Regulatory bodies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) mandate the provision of translated IFU in multiple languages as a prerequisite for market approval. The inclusion of back translation in the translation process serves as compelling evidence of the accuracy and reliability of the translated content, facilitating regulatory compliance and market access.
Risk Mitigation
The consequences of inaccuracies or mistranslations within IFU for medical devices can be far-reaching and potentially catastrophic. From misinterpretation of usage instructions to incorrect dosage information, errors in translated IFU pose a direct threat to patient safety and can expose manufacturers to significant legal liabilities. By implementing rigorous back translation protocols, medical device manufacturers can proactively identify and rectify potential errors, thereby mitigating risks and safeguarding patient well-being.
Best Practices
To maximize the efficacy of the back translation process, adherence to best practices is essential. This encompasses rigorous selection criteria for translators with specialized expertise in medical terminology, fostering clear communication channels between translators and stakeholders, and conducting comprehensive quality assurance checks at each stage of the translation process.
Conclusion
In the translation of “Instructions for Use” in medical devices, the back translation process emerges as an indispensable safeguard against potential inaccuracies and inconsistencies. By incorporating rigorous quality assurance measures, including back translation, medical device manufacturers can uphold the highest standards of accuracy, consistency, and regulatory compliance. In doing so, they reaffirm their unwavering commitment to patient safety and excellence in healthcare delivery.
Ready to ensure the accuracy and safety of your medical device IFU translations? Contact us today to learn how our expert back translation services can support your regulatory compliance and uphold patient safety. Let’s safeguard lives together.






