

Why Choose Hansem Global Life Sciences Localization Services?
Expertise in Life Sciences
Our expert translators have deep knowledge across life science sectors, providing precise translations of complex content, crucial for maintaining the integrity of specialized information across languages.
Regulatory Compliance and Security
Hansem Global meets global standards and regulatory requirements from entities like the EMA and FDA through ISO-certified processes, ensuring compliance and safeguarding sensitive information with the highest security.
Cultural Relevance and Global Reach
We go beyond translation by culturally adapting content to engage specific audiences, enhancing global connections. This ensures translations are linguistically accurate and culturally pertinent, improving engagement and understanding among diverse populations.
Benefits of Localizing Life Science Documents

-
Global Reach
Expand market presence by reaching diverse audiences across regions and languages.
-
Compliance Assurance
Ensure adherence to local regulations and mitigate the risk of non-compliance.
-
Effective Communication
Foster better understanding and collaboration with international partners, healthcare professionals, and patients.
Our Localization Services
eCOA Services
- Translation and Cultural Adaptation
Adapting eCOA content to maintain its intended meaning, relevance, and impact across different languages and cultures. - Migration and Screenshot Review
We streamline the transition of your data from paper to the electronic Clinical Outcome Assessments (eCOA) platform, focusing on accuracy to reduce the need for multiple rounds of screenshot reviews. - Comprehensive Review and Quality Assurance
Rigorous quality checks of all translated content for accuracy, compliance, and cultural appropriateness. - Ongoing Support and Updates
We provide continuous support and updates in line with clinical trial phases and regulatory changes.
 Comprehensive eCOA services for accurate, culturally adapted content
Comprehensive eCOA services for accurate, culturally adapted content
Linguistic Validation
- Medical and Clinical Research Documents
Translating and validating patient-reported outcomes, clinical trial protocols, consent forms, and other research documents. - Cognitive Debriefing
Conducting interviews with target language speakers to assess their understanding and interpretation of translated materials. - Harmonization Across Languages
Guaranteeing consistency in meaning and assessment across multiple languages. - Documentation and Reporting
Providing detailed reports on the validation process, highlighting the methodologies used and the rationale behind linguistic choices.
 Validating medical and clinical documents for global research accuracy
Validating medical and clinical documents for global research accuracy
Pharmaceutical Document Translation
- Hansem Global provides comprehensive pharmaceutical document translation services, catering to essential industry materials.
- Our offerings include translations for Common Technical Documents (CTD/eCTD), Drug Master Files, Toxicology Reports, Chemistry, Manufacturing, and Controls (CMC), Animal Tests, Pharmacovigilance (PV)-related Documents, and regulatory submissions to the FDA, EMA, and NMPA.
- Additionally, we handle translations for Good Manufacturing Practice (GMP) and Standard Operating Procedures (SOP), Master Batch Records, Patents, Packaging Labels and Inserts, as well as Legal and Regulatory Documents. This ensures seamless communication and compliance across global markets.
 Comprehensive localization of pharmaceutical documents for regulatory success
Comprehensive localization of pharmaceutical documents for regulatory success
Clinical Document Translation
- Hansem Global offers specialized translation services for a wide range of clinical documents, ensuring precise and compliant communication essential for global healthcare markets.
- Our services cover Clinical Trial Documentation, Informed Consent Forms (ICF), Clinical Outcome Assessments (COAs), Clinical Trial Agreements, Clinical Study Reports (CSRs), Investigator’s Brochures, Patient Recruitment Materials, and Adverse Events (AEs) including Serious Adverse Events (SAEs), Suspected Unexpected Serious Adverse Reactions (SUSARs), and Case Report Forms (CRFs).
- We also handle translations for Electronic Health Records (EHRs), Development Safety Update Reports (DSURs), and Patient Information Sheets, facilitating accurate and accessible information for all stakeholders.
 Ensuring precision in clinical document localization for global trials
Ensuring precision in clinical document localization for global trials
Research and Medical Device Translation
- Hansem Global provides precise translation services for life science research and medical devices, supporting critical documentation needed for global distribution and regulatory compliance.
- Our offerings include translation of Research Findings, Clinical Study Reports, and Clinical Documents. We also specialize in translating Medical Journals and Articles, Supporting Documentation for CE Submissions, ISO Documents, and Regulatory Submission Dossiers.
- Our expertise extends to Software and Hardware Manuals, Instructions for Use (IFUs), Packaging Inserts, Sensitivity and Biocompatibility Reports, Product Brochures, Quality System Documents, Technical Documents, and Validation Reports.
- This comprehensive service ensures that all medical device documentation meets the stringent standards required for international markets.
 Comprehensive translation solutions for life science research and medical devices
Comprehensive translation solutions for life science research and medical devices
Rely On Our Rigorous Quality Assurance Process
Trust in Hansem Global's stringent quality assurance, which upholds international standards (ISO 9001, ISO 17100, ISO 18587, ISO 27001) to ensure that each translation achieves accuracy, fluency, and cultural appropriateness.
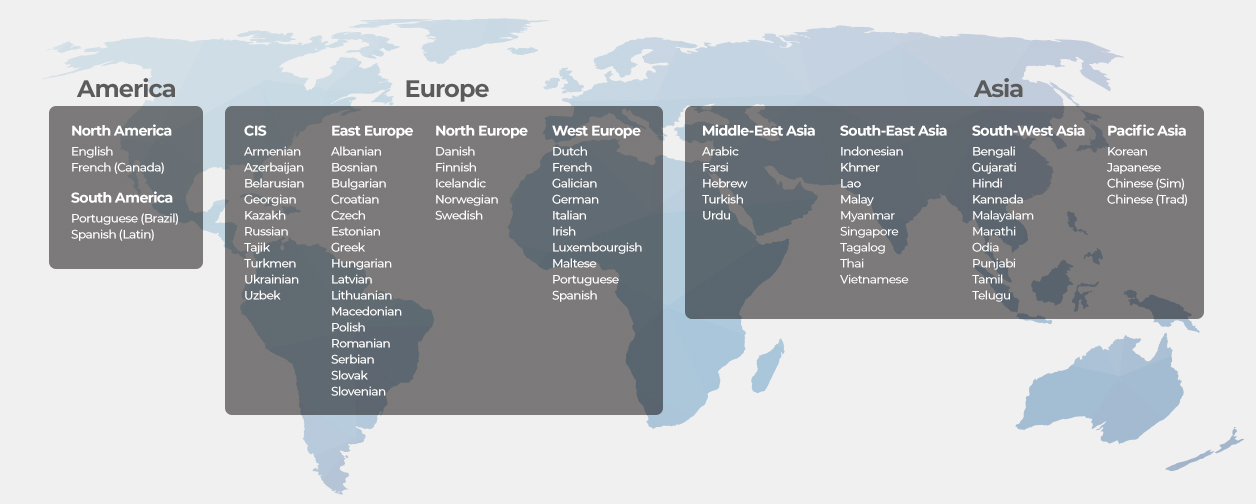
Access the World's Finest Linguists with Hansem Global Language Network

See Our Services In Action
Dive into our collection of client success stories to see the real results we've achieved together.





















